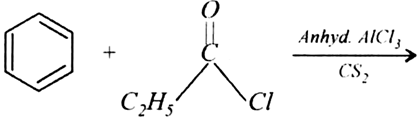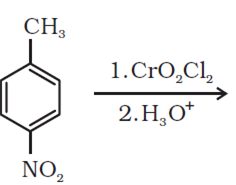Question
Which one of FCH2COOH, ClCH2COOH, BrCH2COOH and ICH2COOH is the strongest acid? Give reason.
Solution
The stronger the electron withdrawing group the greater is the strength of the substituted carboxylic acid. Since fluorine is most electronegative and strongest electron withdrawing group, therefore, fluoroacetic acid F CH2COOH is stronger as compared to chloro, bromo and iodo acetic acids.