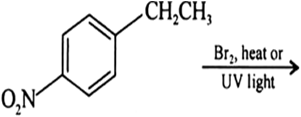
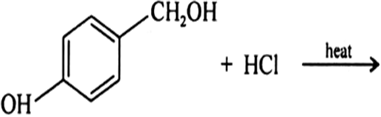Question
Explain why Grignard reagents should be prepared under anhydrous conditions?
Solution
Grignard reagent should be prepared under anhydrous conditions, because it is very reactive. It reacts very quickly with any source of proton to give hydrocarbon. It reacts with water very quickly. Therefore, it is necessary to avoid moisture from the Grignard reagents.
RMgX + H2O → RH + Mg(OH)X
RMgX + H2O → RH + Mg(OH)X