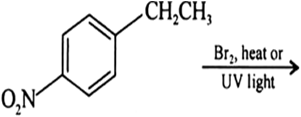
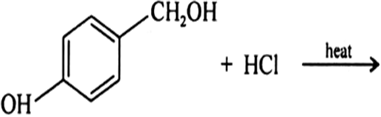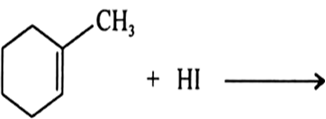Question
Which will have higher boiling point CH3—CH2—CH2—CH2—Br or CH3—CH(CI)— CH3? Give reason.
Solution
Greater the molecular mass of compound more is boiling point. Thus, boiling point of the compound CH3—CH2—CH2—CH2—Br will be high because its molecular mass is higher than the molecular mass of CH3—CH(Cl)—CH3.