Give one chemical test to distinguish between the following pairs of compounds:
(i) Ethylamine and diethylamine.
(ii) Acetaldehyde and benzaldehyde
Chemical test to distinguish between the following pairs:
(i) Ethyl amine and diethylamine
Hinsberg’s test
Ethylamine – when shaken with benzene sulphonyl chloride and aqueous KOH solution, ethyl amine gives a clear solution.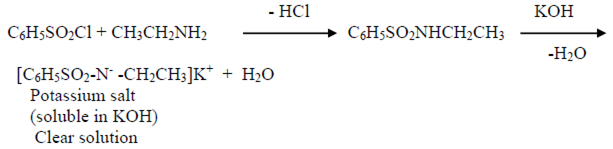
Diethylamine – on similar treatment forms an insoluble substance.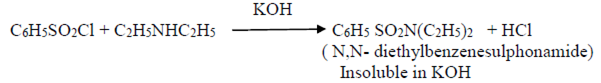
ii) Acetaldehyde to Benzaldehyde
Acetaldehyde gives iodoform test with Iodine and alkali, benzaldehyde does not give iodoform test.





