Dual Nature of Radiation and Matter
Assertion: When a certain wavelength of light fall on a metal surface it ejects electron.
Reason: Light has wave nature.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
B.
If both assertion and reason are true but reason is not the correct explanation of assertion.
Photoelectric emission have been adequately explained by Einstein on the basis of photon theory of light.
Sponsor Area
Some More Questions From Dual Nature of Radiation and Matter Chapter
Wave property of electrons implies that they will show diffraction effects. Davisson and Germer demonstrated this by diffracting electrons from crystals. The law governing the diffraction from a crystal is obtained by requiring that electron waves reflected from the planes of atoms in a crystal interfere constructively (see in the figure).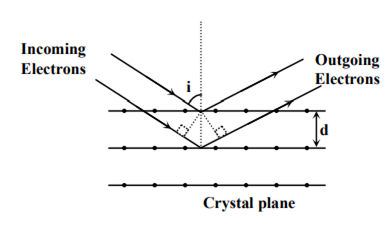
In an experiment, electrons are made to pass through a narrow slit of width’ comparable to their de Broglie wavelength. They are detected on a screen at a distance ‘D’ from the slit (see figure). 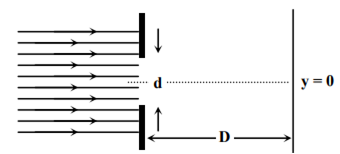
Which of the following graph can be expected to represent the number of electrons ‘N’ detected as a function of the detector position ‘y’(y = 0 corresponds to the middle of the slit)?
The time by a photoelectron to come out after the photon strikes is approximately
An alpha nucleus of energy 1 /2 mv2 bombards a heavy nuclear target of charge Ze. Then the distance of closest approach for the alpha nucleus will be proportional to
If I0 is the intensity of the principal maximum in the single slit diffraction pattern, then what will be its intensity when the slit width is doubled?
The maximum number of possible interference maxima for slit-separation equal to twice the wavelength in Young’s double-slit experiment is
According to Einstein’s photoelectric equation, the plot of the kinetic energy of the emitted photo electrons from a metal Vs the frequency, of the incident radiation gives straight line whose slope
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area