Nuclei
An atom of mass number 15 and atomic number 7 captures an a-particle and then emits a proton. The mass number and atomic number of the resulting atom will be respectively
14 and 12
15 and 3
16 and 4
18 and 8
D.
18 and 8
7X15 + 2He4 → 1H1 + ZYA
Alpha Proton
particle
According to the law of conservation of charge, we get
7 + 2 = 1 + Z
⇒ Z = 8
According to the law of conservation of mass, we get
15 + 4 = 1 + A
⇒ A = 18
Hence, the resulting atom has mass number 18 and atomic number 8.
Sponsor Area
Some More Questions From Nuclei Chapter
The above is a plot of binding energy per nucleon Eb, against the nuclear mass M; A, B, C, D, E, F correspond to different nuclei. Consider four reactions: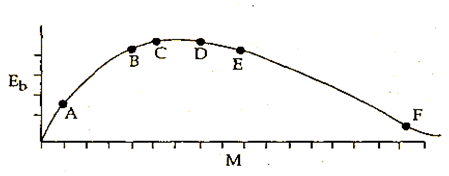
(i) A + B → C + ε (ii) C → A + B + ε
(iii) D + E → F + ε and (iv) F → D + E +ε
where ε is the energy released? In which reaction is ε positive?
This question contains Statement -1 and Statement-2. Of the four choices given after the statements,
Choose the one that best describes the two statements.
Statement – I: Energy is released when heavy nuclei undergo fission or light nuclei undergo fusion.
and
Statement – II: For heavy nuclei, binding energy per nucleon increases with increasing Z while for light nuclei it decreases with increasing Z.
If M0 is the mass of an oxygen isotope 8O17 , Mp and MN are the masses of a proton and a neutron respectively, the nuclear binding energy of the isotope is
In gamma-ray emission from a nucleus
When 3Li7 nuclei are bombarded by protons, and the resultant nuclei are 4Be8 , the emitted particles will be
The ‘rad’ is the correct unit used to report the measurement of
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area