Kinetic Theory
Pressure of an ideal gas is increased by keeping temperature constant. What is effect on kinetic energy of molecules?
Increase
Decrease
No change
Cannot be determined
C.
No change
The kinetic energy of an ideal gas depends only on its temperature. Hence, it remains constant whether its pressure is increased or decreased.
Sponsor Area
Some More Questions From Kinetic Theory Chapter
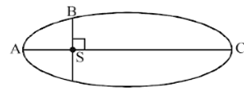
The kinetic energies of a planet in an elliptical orbit about the Sun, at positions A, B and C are KA, KB and KC, respectively. AC is the major axis and SB is perpendicular to AC at the position of the Sun S as shown in the figure. Then
Pressure of an ideal gas is increased by keeping temperature constant. What is the effect on kinetic energy of molecules?
Pressure of an ideal gas is increased by keeping temperature constant. What is effect on kinetic energy of molecules?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area