Periodic Classification of Elements
Taking an example, illustrate a displacement reaction.
The reaction between iron and copper sulphate is a displacement reaction.
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
Here, iron displaces copper from its solution of copper sulphate.
Illustration: Take two test tubes marked as (A) and (B). Add about 10 ml. copper sulphate in each test tube. Now suspend two clean iron nails in tube (B) with the help of a thread [Fig. 1.2(a)], After 20 minutes, take out the iron nails from tube (B). Compare the intensity of the blue colour of copper sulphate solutions in test tubes (A) and (B) [Fig. 1.2 (b)]. It will be observed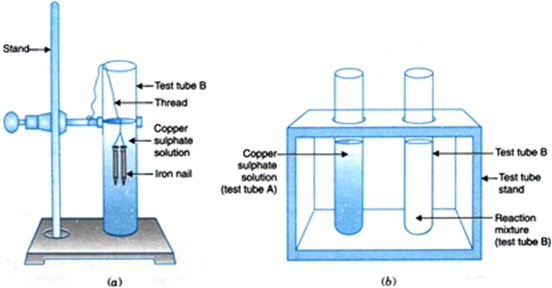
Fig. 1.2. (a) Iron nails dipped in copper sulphate solution (b) Iron nails and copper sulphate solutions compared before (A) and after the experiment (B).
that blue colour of copper sulphate fades in test tube (B) because Cu2+ ions are removed from solution which is deposited on iron nails which then appears brownish. This is due to fine deposition of copper on iron nails.
Sponsor Area
Some More Questions From Periodic Classification of Elements Chapter
What does a balanced chemical equation convey to a chemist?
list the limitations of chemical equation.
What are the essentials of a chemical equation?
What information is available from the following equation:
CaCO3 + 2HCl→ CaCl2 + H2O+ CO2
What is a thermochemical equation? Give two examples.
Write and balance the following equations presented to you as written statements:
(i) Magnesium carbonate plus hydrochloric acid produces magnesium chloride plus water plus carbon dioxide gas.
(ii) Aluminium plus chlorine gas produces aluminium trichloride.
(iii) Nitrogen plus hydrogen produces ammonia.
(iv) Calcium plus sulphur produces calcium sulphide.
(v) Magnesium burns in CO2 to form magnesium oxide and carbon.
H2 + O → H2O is a balanced chemical equation. It is considered to be incorrect. Why?
Complete and balance the following equations:
(i) NaOH +................→ Na2SO4 + H2O
(ii) Ca(OH)2 +................ → CaCO3 + H2O
(iii) ................ + HCl(aq) → MgCl2(aq) +.................
(iv) ................ + Na2SO4(aq) → BaSO4(s) + NaCl(aq)
What are the drawbacks for a chemical equation? Illustrate with an example.
While writing the chemical reaction for the oxidation of calcium in oxygen, a student wrote the following balanced equation:
Ca + O→ CaO
Is it correct? If not, explain and write the correct balanced equation.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area