Matter In Our Surroundings
What is the relation between boiling point of a liquid and the molecular forces of attraction between the particles of a liquid?
Boiling point of liquid depend on the intermolecular force, Stronger the intermolecular forces of attraction, higher would be the boiling point. For example, particles of alcohol have weaker intermolecular forces of attraction than of water particles. This is in conformity with the fact that alcohol boils at lower temperature, of 78°C as compared to boiling point of water which is 100°C.
Sponsor Area
Some More Questions From Matter In Our Surroundings Chapter
Give reason for the following observations.
Naphthalene balls disappear with time without leaving any solid.
Give reason for the following observations.
We can get the smell of perfume sitting several metres away.
What is the physical state of water at
a) 25°C
b)00C
c)1000C
What is the physical state of water at
0°C
What is the physical state of water at
100°C
Give two reasons to justify
Water at room temperature is a liquid.
Give two reasons to justify
An iron almirah is a solid at room temperature.
Why is ice at 273K more effective in cooling than water at the same temperature ?
What produces more severe burns, boiling water or steam ?
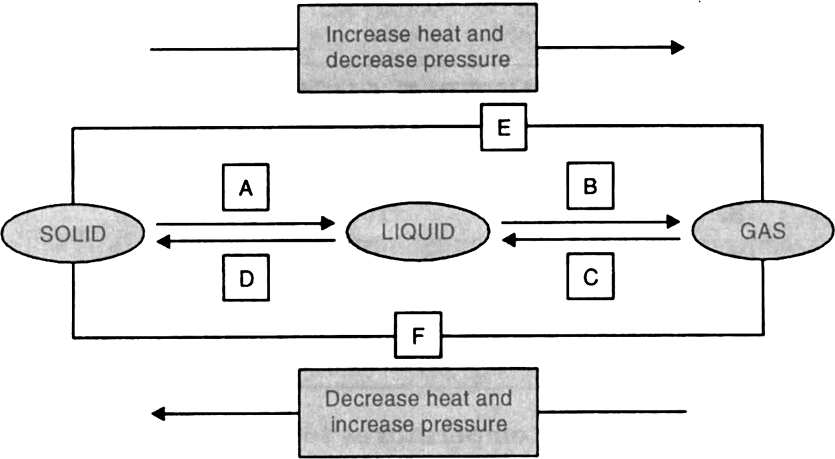
8. Name A, B, C, D, E and F in the following diagram showing state change :
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area