Aldehydes, Ketones and Carboxylic Acids
Question
Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is
-
CH3COOCH3
-
CH3CONH2
-
CH3COOCOCH3
-
CH3COCl
Answer
D.
CH3COCl
Lesser the electron density of acyl carbon atom, more will be the susceptibility of a nucleophile to attack it.
The Cl atom has strong - I effect and weakest +R effect because of the weak π-bond between the small sized C- atom and large sized Cl atom. Thus, in CH3COCl, acyl carbon has least electron density and hence, more susceptible for nucleophilic attack.
Sponsor Area
Some More Questions From Aldehydes, Ketones and Carboxylic Acids Chapter
Write the structures of the following compounds.
2-Hydroxycyclopentane carbaldehyde
Write the structures of the following compounds.
4-oxopentanal
Write the structures of the following compounds.
Di-sec-butyl ketone
Write the structures of the following compounds.
4-fluoro acetophenone.
Write the structures of the following reactions:
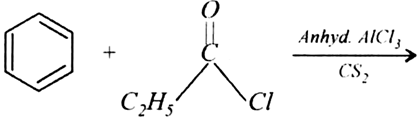
Write the structures of products of the following reactions:
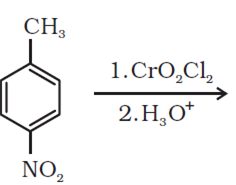
Arrange the following compounds in increasing order of their boiling points:
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3.
Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions:
Ethanal, Propanal, Propanone, Butanone.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area