Alcohols, Phenols and Ethers
Question
Which one of the following compounds has the most acidic nature?
Answer
B.
The presence of electron withdrawing substituent increases the acidity while electron releasing substituent, when present, decrease the acidity.
Phenyl is an electron withdrawing substituent while -CH3 is an electron releasing substituent, Moreover, phenoxide ion is more resonance stabilised as compared to benzoyl oxide ion, thus, release proton more easily. That's why is a strong acid among the given.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Identify allylic alcohols in the above examples. 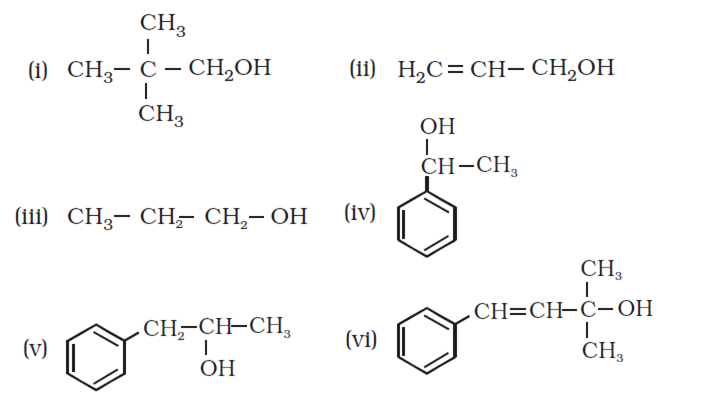
Name the following compounds according to IUPAC system.
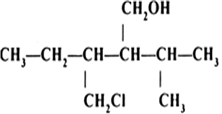
Name the following compounds according to IUPAC system.
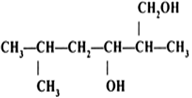
Name the following compounds according to IUPAC system.
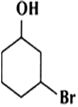
Name the following compounds according to IUPAC system.
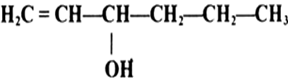
Name the following compounds according to IUPAC system.
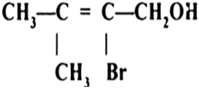
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?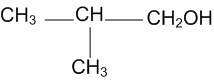
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?

Write structures of the products of the following reactions:
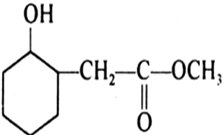
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area