Polymers
Question
The polymer containing strong intermolecular forces e.g. hydrogen bonding is
-
natural rubber
-
Teflon
-
Nylon 6,6
-
Polystyrene
Answer
C.
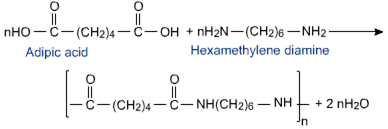
Nylon 6,6
Nylon-6, 6 is a fibre, it contains intermolecular hydrogen bonding. Nylon-6,6 has amide linkage and hydrogen bond are formed between -CONH- a group of successive chains.
Sponsor Area
Some More Questions From Polymers Chapter
Explain the terms polymer and monomer.
How do you explain the functionality of a monomer?
Define the term polymerization.
Is (NH—CHR—CO)n, a homopolymer or copolymer?
What is the basic unit of polymer called?
What is an addition polymer?
What is condensation polymer?
Give one example each of (i) Addition polymer (ii) Condensation polymer.
Give two examples of chain growth polymers.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area