Coordination Compounds
(a) Write the hybridization and shape of the following complexes:
(i) [CoF6]3–
(ii) [Ni (CN)4]2–
(Atomic number: Co = 27, Ni = 28)
(b) Out of NH3 and CO, which ligand forms a more stable complex with a transition metal and why?
(a)
(i) The atomic number of Co is 27 and its valence shell electronic configuration is 3d74s2.
Co is in +3 oxidation state in the complex [CoF6]3-.
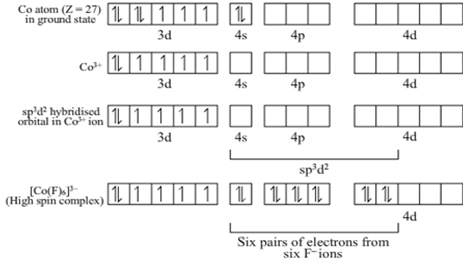
Hence, [CoF6]3- is sp3d2 hybridized and it is octahedral in shape.
(ii) The atomic number of Ni is 28 and its valence shell electronic configuration is 3d84s2.
Ni is in +2 oxidation state in the complex [Ni(CN)4]2–.
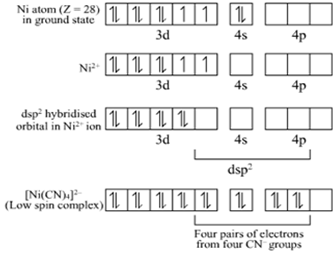
Hence, [Ni(CN)4]2– is dsp2 hybridized and it is square planar in shape.
(b) Out of NH3 and CO, CO ligand forms a more stable complex with transition metal because the metal- carbon bonds in metal carbonyls have both and characters .A bond is formed when carbonyl carbon donates a lone pair of electrons to the vacant orbital of the metal. A bond formed by the donation pair of electrons from filled metal d orbital into the vacant anti- bonding orbital ( also known as back bonding of the carbonyl group). And sigma bond strengthen the pi bond vice-versa. Thus, a synergic effect s created due to this metal- ligand bonding. These synergic effects strengthen the bond between CO and the metal.
Sponsor Area
Some More Questions From Coordination Compounds Chapter
Write the formula for the following coordination compound:
Potassium tetracyanidonickelate(II)
Write the formula for the following coordination compound:
Tris(ethane -1, 2-diamine) chromium(III) chloride
Write the formula for the following coordination compound:
Amminebromidochloridonitrito-N-platinate (II)
Write the formula for the following coordination compound:
Dichloridobis(ethane-1, 2-diamine)platinum(lV) nitrate.
Write the formula for the following coordination compound:
Iron(III) hexacyanidoferrate(II)
Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl(NH2CH3)]Cl.
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers:
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with square planar is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area