Coordination Compounds
Question
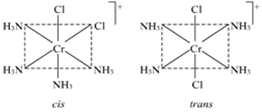
Write the IUPAC name of the complex [Cr(NH3)4Cl2]+. What type of isomerism does it exhibit?
Answer
The IUPAC name of the complex [Cr(NH3)4Cl2]+ is Tetraamminedichlorochromium(III) ion.
This complex exhibits geometrical isomerism. [Cr(NH3)4Cl2]+ is a [MA4B2] type of complex, in which the two chloride ligands may be oriented cis and trans to each other.
Sponsor Area
Some More Questions From Coordination Compounds Chapter
Write the formula for the following coordination compound:
Amminebromidochloridonitrito-N-platinate (II)
Write the formula for the following coordination compound:
Dichloridobis(ethane-1, 2-diamine)platinum(lV) nitrate.
Write the formula for the following coordination compound:
Iron(III) hexacyanidoferrate(II)
Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl(NH2CH3)]Cl.
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers:
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with square planar is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area