Alcohols, Phenols and Ethers
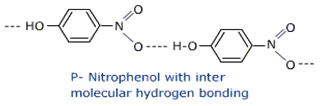
Which of the following isomers is more volatile: o-nitrophenol or p-nitrophenol?
In o-nitro phenol, -OH is linked to –NO2 by means of intramolecular H-bonding so, it is highly volatile.
Explanation:
Stronger intermolecular forces would make the substance less volatile. So we can see intermolecular H-bonding in P-nitro phenol, so P-nitro phenol is less volatile, where as in o-nitro phenol intra H-bonding takes places that’s why it is highly volatile.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Classify the following as primary, secondary and tertiary alcohols: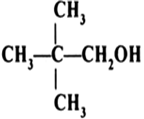
Classify the following as primary, secondary and tertiary alcohols: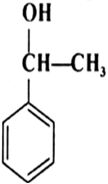
Classify the following as primary, secondary and tertiary alcohols: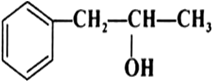
Classify the following as primary, secondary and tertiary alcohols: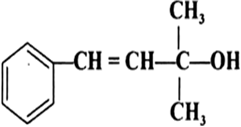
Identify allylic alcohols in the above examples. 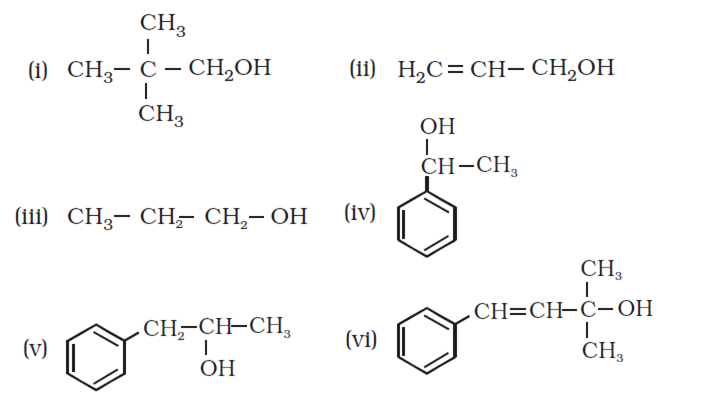
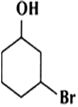
Name the following compounds according to IUPAC system.
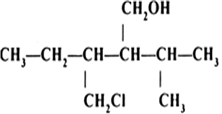
Name the following compounds according to IUPAC system.
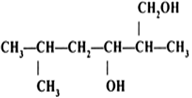
Name the following compounds according to IUPAC system.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area