General Principles And Processes of Isolation Of Elements
Is it true that under certain conditions. Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?
Yes, below 1350°C Mg can reduce Al2O3 and above 1350°C. Al can reduce MgO. This can be inferred from ΔG° Vs T plots.
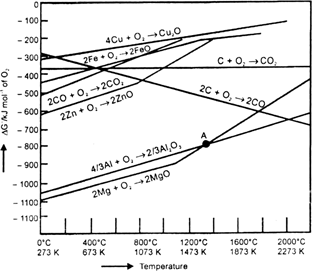
Fig. Gibbs energy (ΔG°) V T plots for formation of some oxides.
Sponsor Area
Some More Questions From General Principles And Processes of Isolation Of Elements Chapter
What criterion is followed for the selection of the stationary phase in chromatography?
What is the role of cryolite in the metallurgy of aluminium?
Why is zinc not extracted from zinc oxide through reduction using CO?
What is the role of graphite rod in the electrometallurgy of aluminium?
Predict conditions under which Al might be expected to reduce MgO.
Define the term gangue or matrix.
Define the term flux.
Deine the term slag.
Why magnesium oxide is used for the lining in steel making furnance?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area