Chemistry in Everyday Life
What are detergents? Give their scheme of classification. Why are detergents preferred over soaps?
Detergents are sodium salts of long chain sulphonates and sulphates.
Classification: They are two types:
(i) Sodium salts of long chain benzene sulphonic acid: These are obtained from derivatives of benzene sulphonic acid. The common example is sodium p-dodecyl benzene sulphonate.
(ii) Sodium salts of long chain alkyl hydrogen sulphate: These are sodium salts of sulphuric acid esters of long-chain alcohols containing usually 10-15 carbon atoms. These alcohols are obtained by the hydrogenolysis of oils and fats. For examples, sodium dodecyl sulphate or sodium lauryl sulphate and sodium cetyl sulphate.
Detergents are preferred our soaps for the following reasons:
(i) Synthetic detergents can be used even in the case of hard water whereas soaps fail to do so.
(ii) Synthetic detergents can be used in the acidic medium while soaps fail to do so because of their hydrolysis to free acids.
(iii) Synthetic detergents are more soluble in water and hence form more latter than soaps.
(iv) Synthetic detergents have a stronger cleansing action than soaps.
Sponsor Area
Some More Questions From Chemistry in Everyday Life Chapter
Write the chemical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structure of these compounds are given below:
![]()
Following type of non-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic parts in the molecule. Identify the functional group(s) present in the molecule.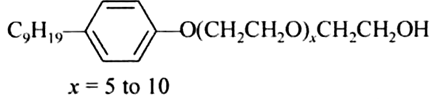
Define the term chemotherapy?
What is drug?
Name the macro-molecules that are chosen as drug targets.
Why should not medicines be taken without consulting doctors?
Name a substance which can be used as an antiseptic as well as disinfectant.
What are the main constitutents of dettol?
What is tincture of iodine? What is its use?
What are food preservatives?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area