Chemistry in Everyday Life
Describe the following with suitable examples:
(i) Preservative
(ii) Artificial sweeteners
(iii) Antioxidants
(iv) Edible colours.
(i) Preservatives: These are the chemical substances which are added to the food materials to prevent their spoilage and to retain their nutritive value for long periods. These preservatives prevent the rancidity of food and inhibit and growth or kill the micro-organisms.
Examples: Salt, sugar, sodium nitrate/nitrite, sodium benzoate, sodium metabisulphite, sorbic acid, potassium sorbate, Na and Ca propionate.
(ii) Artificial sweeteners : These are the chemical compounds which give sweetening effect to the food and enhance its odour and flavour.
Examples : Saccharin, aspartame (methyl ester), nitro aniline, dulcin, (urea, sweetener), dihydro-chalcones (DHC), sucralose etc.
(iii) Antioxidants: An antioxidant may be defined as the substance which when added to the fats and fat-containing foods prevent their oxidation and thus prolong their life. The commonly used antioxidants are Butylated hydroxy anisole (BHA), Butylated hydroxy toluene (BHT), Propyl gallate (PG), Tertiary butyl hydroquinone (TBHQ).
(iv) Edible colours: These are the chemical substances which are used for imparting colour to the food and increase the eye appeal and compliment a definite flavour. The main condition for using a colour in food is that it should have less to the human health. It should be stable towards the action of acids, alkalies, high temperature, day light and the long storage. The use of the following dyes has been permitted to impart characteristics colours.
Azo dyes: Red colour
Pyrazolone dye: Yellow
Indigoid: Blue
Triphenyl methane: Green
According to the Public Health Department, the following colouring matters are not allowed in food products.
Metallic colours: Compounds of metals like antimony; arsenic, cadmium, chromium, copper, mercury, lead and zinc.
Vegetable colouring matter: Garbage.
Coal tar colours: Cabazotic acid, dinitrocresol, Naphthol yellow, Martius yellow, etc.
Sponsor Area
Some More Questions From Chemistry in Everyday Life Chapter
Write the chemical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structure of these compounds are given below: ![]()
Write the chemical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structure of these compounds are given below:
![]()
Following type of non-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic parts in the molecule. Identify the functional group(s) present in the molecule.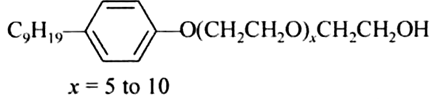
Define the term chemotherapy?
What is drug?
Name the macro-molecules that are chosen as drug targets.
Why should not medicines be taken without consulting doctors?
Name a substance which can be used as an antiseptic as well as disinfectant.
What are the main constitutents of dettol?
What is tincture of iodine? What is its use?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area