Polymers
Write the free radical mechanism for the polymerization of ethene.
Free redical mechanism involves three steps, such as ;
i) chain initiating step
ii) chain propagating step
iii)chain terminating step
In the polymerisation of ethene to polythene. Ethene is heating or exposing to light with benzoyl peroxide initiator. In this process a larger free radical is form.
As this radical reacts with another molecule of ethene, another bigger sized readical is formed. The repetition of this sequence is known as chain propagating step.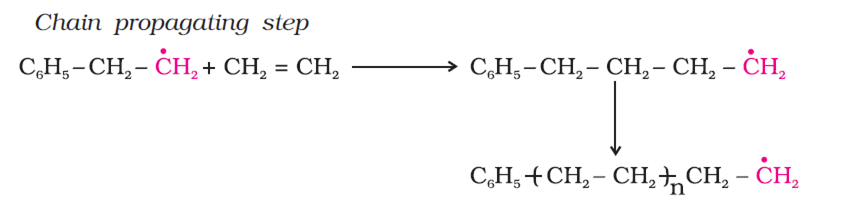
For the treminating of the long chain, these free radical can combine on different ways to form polythene.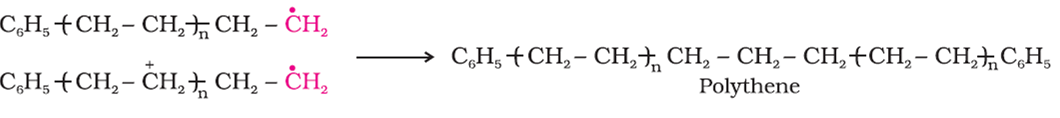
Sponsor Area
Some More Questions From Polymers Chapter
Explain the difference between Buna-N and Buna-S.
Arrange the following polymers in increasing order of their intermolecular forces.
Nylon 6, 6, Buna-S, Polythene.
Arrange the following polymers in increasing order of their intermolecular forces.
Nylon-6, Neoprene, Polyvinyl chloride.
Explain the terms polymer and monomer.
How do you explain the functionality of a monomer?
Define the term polymerization.
Is (NH—CHR—CO)n, a homopolymer or copolymer?
What is the basic unit of polymer called?
What is an addition polymer?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area