Amines
Question
Why is an amide more acidic than an amine?
Answer
Amides are less basic than amine because unlike amines the lone pair of nitrogen in amide is not always available with nitrogen due to the following resonating structures:
In structure (II), a positive charge is developed on nitrogen because of which it can liberate a proton and shows acidic character.
2CH3CONH2 + HgO → (CH3CONH)2 + HgO
Sponsor Area
Some More Questions From Amines Chapter
Classify the following amines as primary, secondary or tertiary:
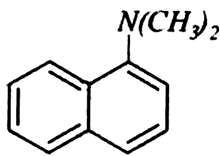
Classify the following amines as primary, secondary or tertiary:
(C2H5)2CHNH2
Classify the following amines as primary, secondary or tertiary:
(C2H5)2NH
Write structures of different isomeric amines corresponding to the molecular formula C4H11N.
Write IUPAC names of all the isomers.
What type of isomerism is exhibited by different pairs of amines?
How will you convert Benzene into N, N-dimethylaniline?
Arrange the following in increasing order of their basic strength:
C2H5NH2, C6H5NH2, NH3, C6H5CH2NH2 and (C2H5)2NH
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area