Amines
Question
Why does bromination of aniline, even under very mild conditions give 2, 3, 5-tribromo aniline instantaneously?
Answer
NH2-group of aniline greatly activates the benzene ring, because of which the electron density at ortho positions correct (at 2 and 6) and para position (at 4 positions) increases appreciably. Thus, under very mild conditions, even in the absence of a catalyst, aniline instantaneously get brominated at both the ortho position and para position to give a white precipitate.
Sponsor Area
Some More Questions From Amines Chapter
Classify the following amines as primary, secondary or tertiary:
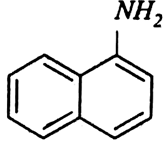
Classify the following amines as primary, secondary or tertiary:
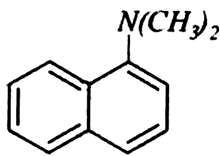
Classify the following amines as primary, secondary or tertiary:
(C2H5)2CHNH2
Classify the following amines as primary, secondary or tertiary:
(C2H5)2NH
Write structures of different isomeric amines corresponding to the molecular formula C4H11N.
Write IUPAC names of all the isomers.
What type of isomerism is exhibited by different pairs of amines?
How will you convert Benzene into N, N-dimethylaniline?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area