Aldehydes, Ketones and Carboxylic Acids
Sponsor Area
Some More Questions From Aldehydes, Ketones and Carboxylic Acids Chapter
Write the structures of the following compounds.
3-Hydroxybutanal
Write the structures of the following compounds.
2-Hydroxycyclopentane carbaldehyde
Write the structures of the following compounds.
4-oxopentanal
Write the structures of the following compounds.
Di-sec-butyl ketone
Write the structures of the following compounds.
4-fluoro acetophenone.
Write the structures of the following reactions:
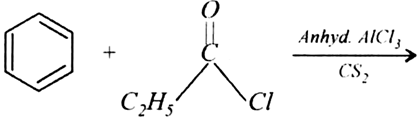
Write the structures of products of the following reactions:
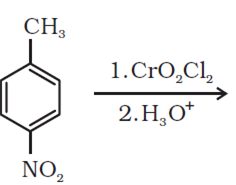
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area