Aldehydes, Ketones and Carboxylic Acids
Question
Which is a stronger acid and why? Nitroacetic acid or chloroacetic acid?
Answer
Electron withdrawing group increase the acidity of carboxylic acid by stabilising conjugate base through delocalisation of the negative charge. Both nitro and chloro group are electron withdrawing group. O2NCH2COOH is a stronger acid than ClCH2COOH because NO2 is a stronger electron withdrawing group than Cl due to +ve charge on N.
Sponsor Area
Some More Questions From Aldehydes, Ketones and Carboxylic Acids Chapter
Write the structures of the following compounds.
3-Hydroxybutanal
Write the structures of the following compounds.
2-Hydroxycyclopentane carbaldehyde
Write the structures of the following compounds.
4-oxopentanal
Write the structures of the following compounds.
Di-sec-butyl ketone
Write the structures of the following compounds.
4-fluoro acetophenone.
Write the structures of the following reactions:
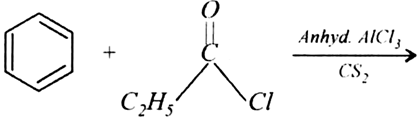
Write the structures of products of the following reactions:
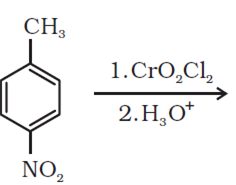
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area