Alcohols, Phenols and Ethers
Draw resonating structures for phenol showing the correct position of positive and negative charges and then account for the following:
(i) The ring of phenol is more easily oxidised than that of benzene.
(ii) Phenol is nitrated more easily than benzene.
The resonating structures for phenol are:
(i) The OH group is strongly activating group and increases the electron density on benzene and making the ring of phenol very electron rich. As a result, it can readily donate electrons to an oxidising agents.
(ii) Nitration of phenol is an electrophilic substitution reaction. OH group increases the electron density on benzene ring making it more electron rich. As a result, NO2+ ion can easily attack on the ring of phenol.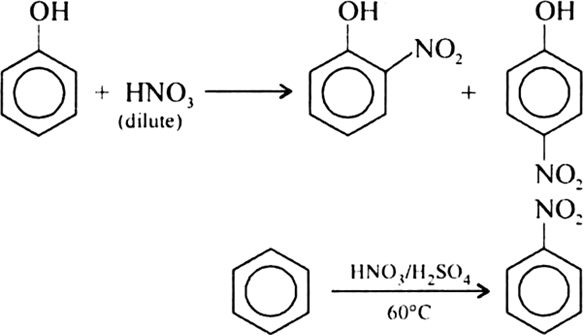
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Classify the following as primary, secondary and tertiary alcohols: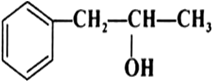
Classify the following as primary, secondary and tertiary alcohols: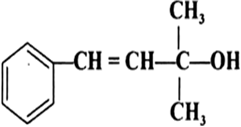
Identify allylic alcohols in the above examples. 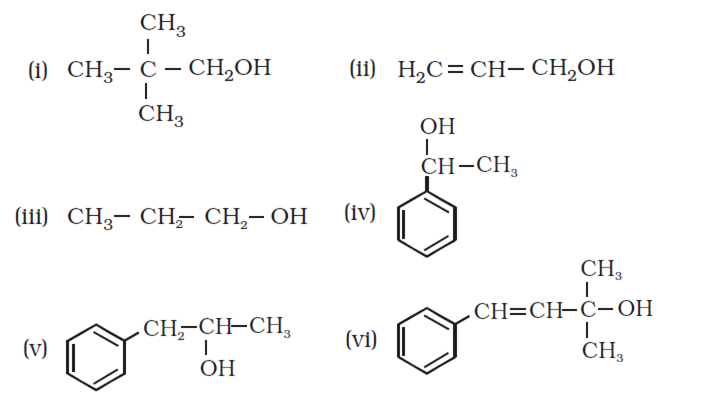
Name the following compounds according to IUPAC system.
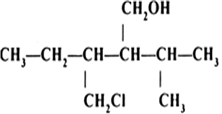
Name the following compounds according to IUPAC system.
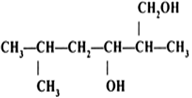
Name the following compounds according to IUPAC system.
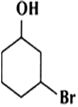
Name the following compounds according to IUPAC system.
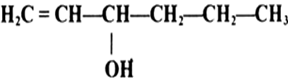
Name the following compounds according to IUPAC system.
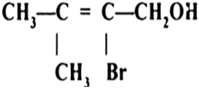
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?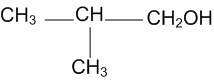
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?

Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area