Alcohols, Phenols and Ethers
Question
Write the resonance structures of phenol (which is similar to that of chlorobenzene) and explain why it does not form chlorobenzene on reaction with PCI5 Explain whether phenol can be dehydrated on heating with catalytic amounts of conc. H2SO4.
Answer
Resonance structures of phenol:
Due to resonance, carbon-oxygen bond develops a slight double bond character. Therefore, it is not easy to replace —OH in phenols by Cl.
No, phenols cannot be dehydrated on heating with small amount of conc. H2SO4. Instead, phenols with conc. H2SO4 undergoes sulphonation.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Name the following compounds according to IUPAC system.
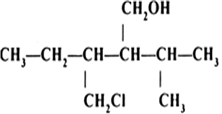
Name the following compounds according to IUPAC system.
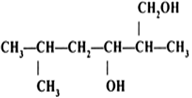
Name the following compounds according to IUPAC system.
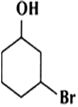
Name the following compounds according to IUPAC system.
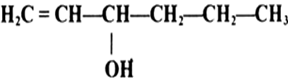
Name the following compounds according to IUPAC system.
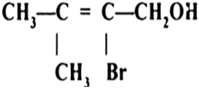
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?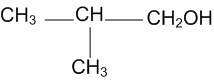
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?

Write structures of the products of the following reactions:
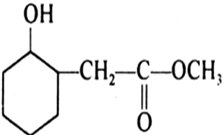
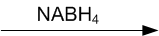
Write structures of the products of the following reactions:
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area