Alcohols, Phenols and Ethers
Explain giving reasons, why:
Phenol has a smaller dipole moment than methanol?
Phenol has a smaller dipole moment (1.54 D) than methanol. The smaller dipole moment of phenol is due to the electron withdrawing effect of phenyl group (whereas alkyl group in alcohols has electron releasing effect).
In phenol, the lone pairs of electrons of the oxygen atom are delocalised across the aromatic ring (via electron transfer known as resonance) this reduces the polarity of the C-O bond. Methanol, which has no aromatic ring and the lone pair of electrons from the oxygen are not delocalised meaning that the C-O bond, in this case, has a higher polarity.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Name the following compounds according to IUPAC system.
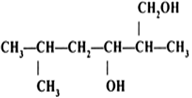
Name the following compounds according to IUPAC system.
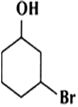
Name the following compounds according to IUPAC system.
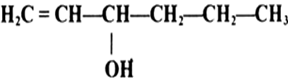
Name the following compounds according to IUPAC system.
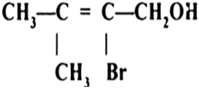
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?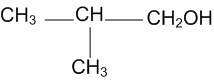
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?

Write structures of the products of the following reactions:
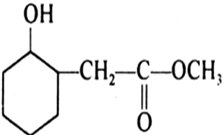
Write structures of the products of the following reactions:
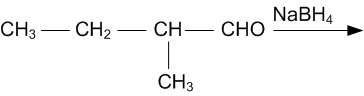
Give structures of the products you would expect when each of the following alcohol reacts with,
(a) HCl—ZnCl2 (b) HBr and (c) SOCl2.
(i) Butan-1-ol (ii) 2-Methyl butan-2-ol.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area