Alcohols, Phenols and Ethers
Question
How many σ-bonds are present in 3-methyl phenol?
Answer
6 carbon atoms of the ring form a sigma bond with the neighbouring carbon atoms of the ring. Therefore 6 sigma bonds. Another carbon atom forms a sigma bond with the carbon of -CH3 group which in turn forms a sigma bond with each of the 3 hydrogen atoms. 4 more sigma bonds.
4 carbon atoms of the ring form a sigma bond with hydrogen atoms. 4 more sigma bonds.
-OH form 2 sigma bond.
Total =6+2+4+4 =16 sigma bonds.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Name the following compounds according to IUPAC system.
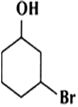
Name the following compounds according to IUPAC system.
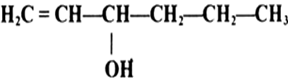
Name the following compounds according to IUPAC system.
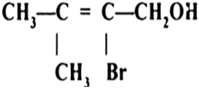
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?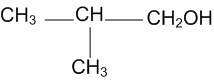
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?

Write structures of the products of the following reactions:
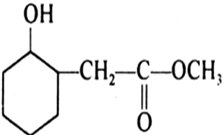
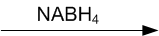
Write structures of the products of the following reactions:
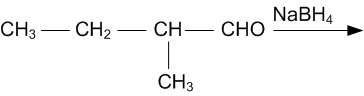
Give structures of the products you would expect when each of the following alcohol reacts with,
(a) HCl—ZnCl2 (b) HBr and (c) SOCl2.
(i) Butan-1-ol (ii) 2-Methyl butan-2-ol.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area