Alcohols, Phenols and Ethers
Write one example of each:
(i) a nucleophile, (ii) an electrophile.
All molecules or ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases. For example Cynaide ion CN- or Hydroxyl ion OH- are nucleophilies.
Electrophiles are positively charged or neutral species having vacant orbitals that are attracted to an electron rich centre. It participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile. Because electrophiles accept electrons, they are Lewis acids. For example mercury cation Hg+.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Classify the following as primary, secondary and tertiary alcohols: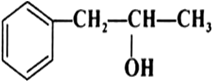
Classify the following as primary, secondary and tertiary alcohols: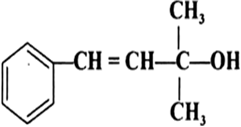
Identify allylic alcohols in the above examples. 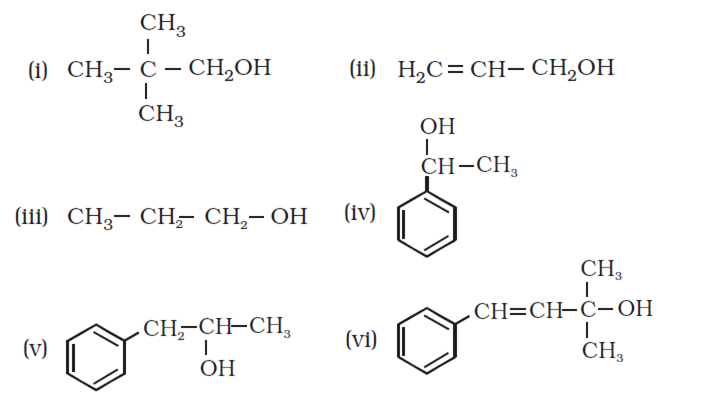
Name the following compounds according to IUPAC system.
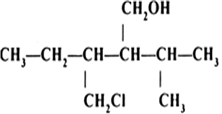
Name the following compounds according to IUPAC system.
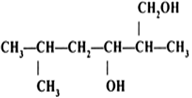
Name the following compounds according to IUPAC system.
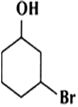
Name the following compounds according to IUPAC system.
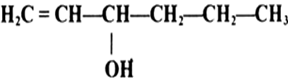
Name the following compounds according to IUPAC system.
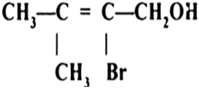
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?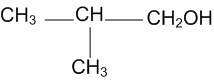
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?

Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area