Alcohols, Phenols and Ethers
Which of the following will have a higher boiling point and why? CH3NH2 or CH3OH.
Molecular mass of methanol is 32g/mol and molecular mass of methylamine is 31g/mol. Since methanol have higher molecular mass thus it have higher boiling point. Also, CH3OH has very strong H-bonding between its molecules. This is due to the greater electronegativity of oxygen atom. Such strong association of molecules leads to rise in the boiling point. CH3NH2 has comparitively lesser H-bonding due to lesser electronegativity of N atom and hence lesser boiling atom.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Classify the following as primary, secondary and tertiary alcohols: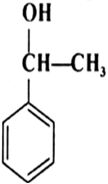
Classify the following as primary, secondary and tertiary alcohols: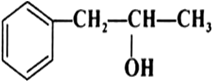
Classify the following as primary, secondary and tertiary alcohols: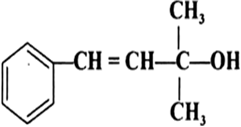
Identify allylic alcohols in the above examples. 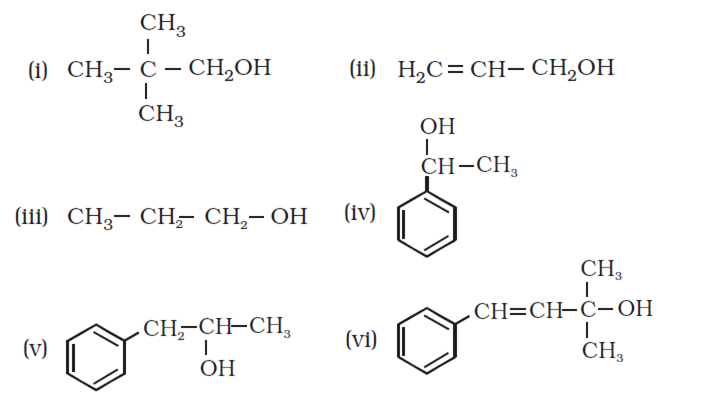
Name the following compounds according to IUPAC system.
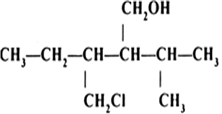
Name the following compounds according to IUPAC system.
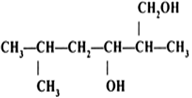
Name the following compounds according to IUPAC system.
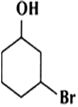
Name the following compounds according to IUPAC system.
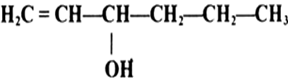
Name the following compounds according to IUPAC system.
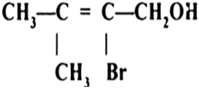
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?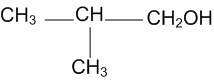
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area