Alcohols, Phenols and Ethers
Explain why is ortho-nitrophenol more acidic than ortho-methoxy phenol?
Ortho nitrophenol is more acidic than ortho-methoxyphenol. This is because the nitro-group is an electron-withdrawing group. The presence of this group in the ortho position in ortho nitro phenol decreases the electron density in the O−H bond. As a result, it is easier to lose a proton.Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid.
In the case of ortho methoxyphenol; methoxy group is an electron-releasing group. Thus, it increases the electron density in the O−H bond and hence, the proton cannot be given out easily. Hence, ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Classify the following as primary, secondary and tertiary alcohols: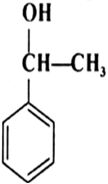
Classify the following as primary, secondary and tertiary alcohols: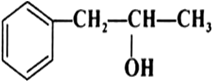
Classify the following as primary, secondary and tertiary alcohols: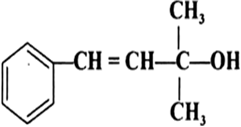
Identify allylic alcohols in the above examples. 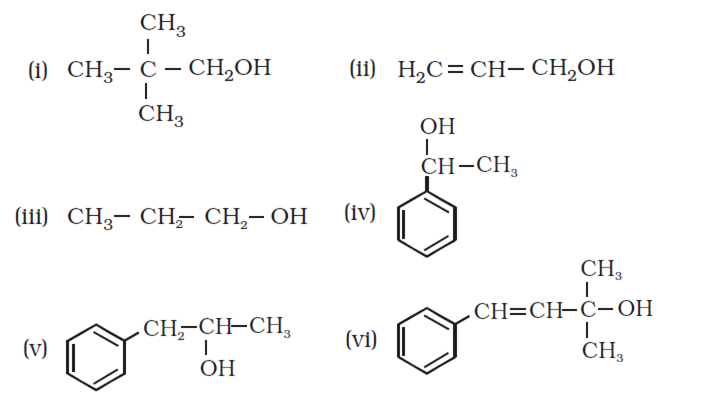
Name the following compounds according to IUPAC system.
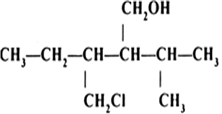
Name the following compounds according to IUPAC system.
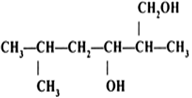
Name the following compounds according to IUPAC system.
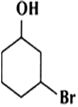
Name the following compounds according to IUPAC system.
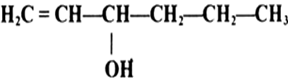
Name the following compounds according to IUPAC system.
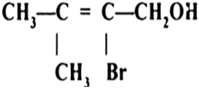
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?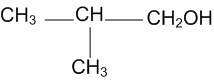
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area