Alcohols, Phenols and Ethers
Compare the acidic strength:
(i) 2, 4-dinitrophenol and 2,4,6-trinitro phenol.
(ii) O-amino phenol and m-amino phenol.
(i) 2, 4, 6 trinitro phenol (picric acid) is stronger acid than 2, 4-dinitrophenol as it contains three electron withdrawing NO2 groups.
(ii) NH3 group is electron donating by resonance when present at ortho and para position while it has -I effect when present at m-position (lone pair can not participate into resonance with benzene from m-position. In m-amino phenol, therefore, the electron density around H of O—H group decreases and H can easily be removed as H+ ion. Hence, m-amino phenol is more acidic than o-amino phenol.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Classify the following as primary, secondary and tertiary alcohols: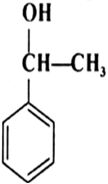
Classify the following as primary, secondary and tertiary alcohols: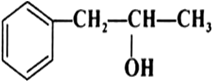
Classify the following as primary, secondary and tertiary alcohols: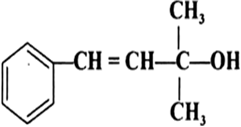
Identify allylic alcohols in the above examples. 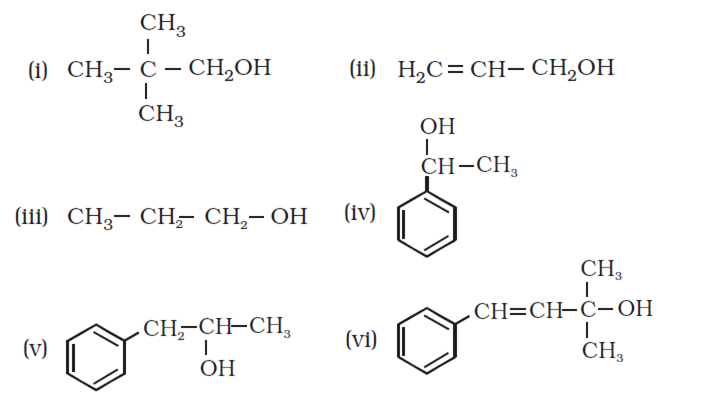
Name the following compounds according to IUPAC system.
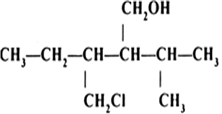
Name the following compounds according to IUPAC system.
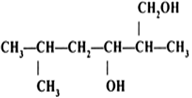
Name the following compounds according to IUPAC system.
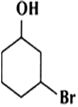
Name the following compounds according to IUPAC system.
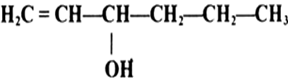
Name the following compounds according to IUPAC system.
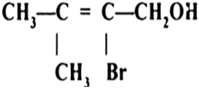
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area