Alcohols, Phenols and Ethers
Write mechanism of acid dehydration of ethanol to yield ethene.
Mechanism: The formation of ethylene and diethyl ether from ethanol and conc. H2SO4 may be explained as follows:
(a) Formation of ethene or ethylene:
Step 1. Due to the presence of two lone pairs of electrons on oxygen, alcohols act as weak bases. Therefore, they react with strong mineral acids (HCl, H2SO4 etc.) to form oxonium salts.
Step 2. The presence of a positive charge on the highly electronegative oxygen atom weakens the C—O bond. Thus, the protonated ethanol readily eliminates a molecule of H2O to form ethyl carbocation.
This step is slow and hence is the rate-determining step of the reaction.
Step 3. The ethyl carbocation formed in step 2 being a reactive chemical species, readily loses a proton to form the ethene molecule.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Name the following compounds according to IUPAC system.
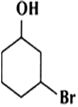
Name the following compounds according to IUPAC system.
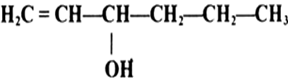
Name the following compounds according to IUPAC system.
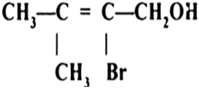
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?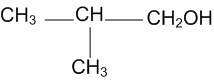
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?

Write structures of the products of the following reactions:
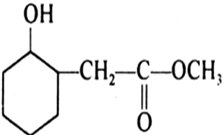
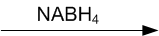
Write structures of the products of the following reactions:
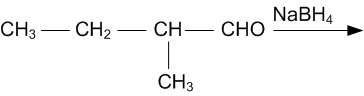
Give structures of the products you would expect when each of the following alcohol reacts with,
(a) HCl—ZnCl2 (b) HBr and (c) SOCl2.
(i) Butan-1-ol (ii) 2-Methyl butan-2-ol.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area