Alcohols, Phenols and Ethers
Question
Ethanol and chloro ethane both are polar in nature yet ethanol is miscible in water while chloro ethane is immiscible. Explain.
Answer
Ethanol is miscible in water due to its ability to form hydrogen bonds with water.
Chloroethane does not form hydrogen bonds with water, a result it is immiscible.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Classify the following as primary, secondary and tertiary alcohols: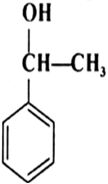
Classify the following as primary, secondary and tertiary alcohols: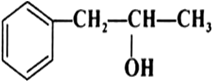
Classify the following as primary, secondary and tertiary alcohols: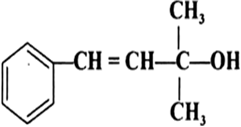
Identify allylic alcohols in the above examples. 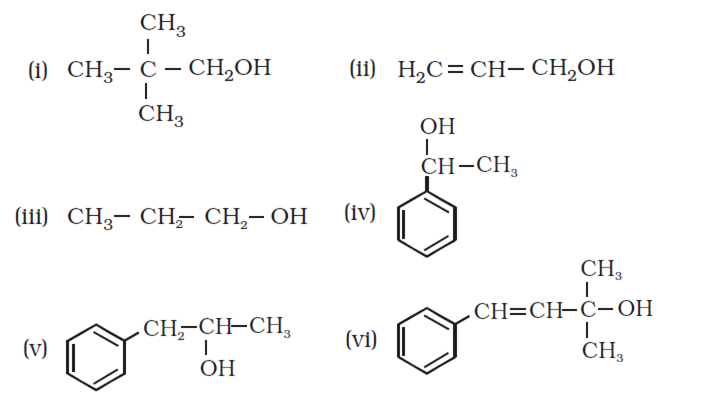
Name the following compounds according to IUPAC system.
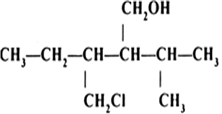
Name the following compounds according to IUPAC system.
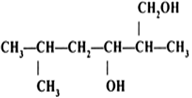
Name the following compounds according to IUPAC system.
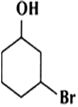
Name the following compounds according to IUPAC system.
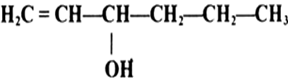
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area