Alcohols, Phenols and Ethers
Question
Phenol is acidic but does not decompose NaHCO3 solution while carbonic acids like CH3COOH decompose NaHCO3 solution. Why?
Answer
Phenol is weaker acid than carbonic acids, Ka value of phenol is very less than that of carbonic acids like CH3COOH, C6H5COOH etc. Therefore phenol does not decompose NaHCO3 solution.
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Classify the following as primary, secondary and tertiary alcohols: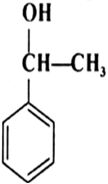
Classify the following as primary, secondary and tertiary alcohols: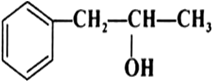
Classify the following as primary, secondary and tertiary alcohols: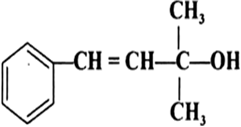
Identify allylic alcohols in the above examples. 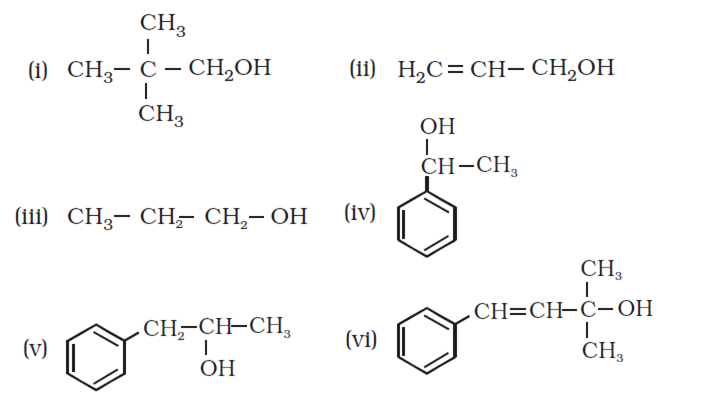
Name the following compounds according to IUPAC system.
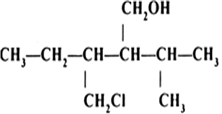
Name the following compounds according to IUPAC system.
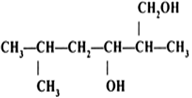
Name the following compounds according to IUPAC system.
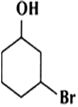
Name the following compounds according to IUPAC system.
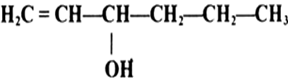
Name the following compounds according to IUPAC system.
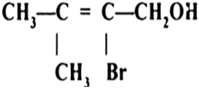
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area