Alcohols, Phenols and Ethers
Question
Explain why is propanol has higher boiling point than that of hydrocarbon, butane?
Answer
Propanol has higher boiling point than butane because of wide spread H-bonding among propanol molecules. A lot of energy is required to break inter molecular H-bonding among propanol molecules. There is no such bonding in butane.
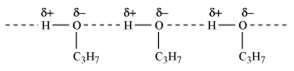
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Name the following compounds according to IUPAC system.
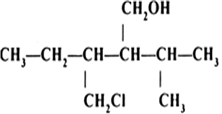
Name the following compounds according to IUPAC system.
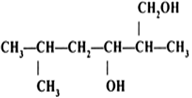
Name the following compounds according to IUPAC system.
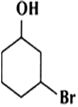
Name the following compounds according to IUPAC system.
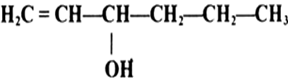
Name the following compounds according to IUPAC system.
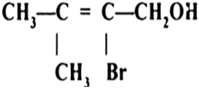
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?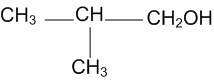
Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?

Write structures of the products of the following reactions:
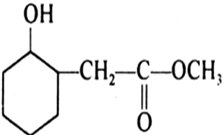
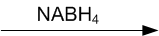
Write structures of the products of the following reactions:
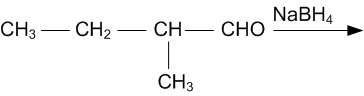
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area