Alcohols, Phenols and Ethers
Question
Give structures of the products you would expect when each of the following alcohol reacts with,
(a) HCl—ZnCl2 (b) HBr and (c) SOCl2.
(i) Butan-1-ol (ii) 2-Methyl butan-2-ol.
Answer
(HCl—ZnCl2) is lucas reagent, lucas reagent react with alcohol however it does not react with primary alcohol but readily gives turbidity with tertiary alcohols.
a) Reaction with (HCl—ZnCl2):
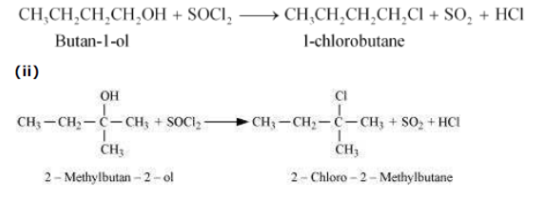
Butan-1-ol is primary alcohol thus no reaction occur.
where as 2-methyl butan-2-ol is tertiary alcohol, it forms 2-chloro-2-methylbutane.
b) Reaction with HBr:
c) Reaction with SOCl2:
Sponsor Area
Some More Questions From Alcohols, Phenols and Ethers Chapter
Classify the following as primary, secondary and tertiary alcohols: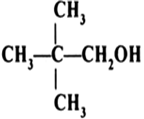
Classify the following as primary, secondary and tertiary alcohols: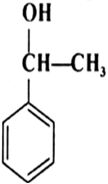
Classify the following as primary, secondary and tertiary alcohols: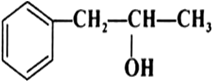
Classify the following as primary, secondary and tertiary alcohols: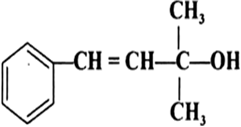
Identify allylic alcohols in the above examples. 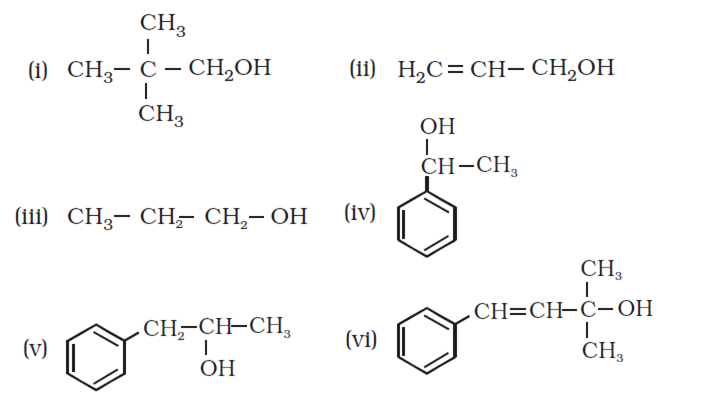
Name the following compounds according to IUPAC system.
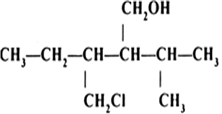
Name the following compounds according to IUPAC system.
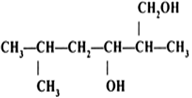
Name the following compounds according to IUPAC system.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area