The p-Block Elements
Question
(Oxides of nitrogen have open chain structures while those of phosphorus have closed chain or cage structures). Why is it so? Illustrate with one structural example for each type of oxides.
Answer
Nitrogen is small in size and have ability to form multiple bonding with oxygen.thus oxides of nitrogen have open chain structures. For example N2O5.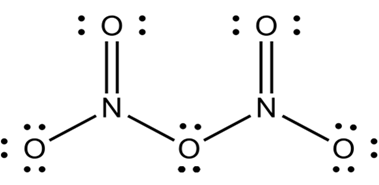
On other hand phosphorus due to its larger size does not form this type of multiple bonds with oxygen but instead forms single bonds and forms oxides with cage like structure.
For example P4O10.
Sponsor Area
Some More Questions From The p-Block Elements Chapter
Write a balanced equation for the hydrolytic reactions PCl5 in heavy water.
What is the basicity of H3PO4?
What happens when H3PO3 is heated?
List the important source of sulphur.
Write the order of thermal stability of the hydrides of Group 16 elements.
Why is H2O a liquid and H2S a gas?
Which of the following does not react with oxygen directly?
Zn, Ti, Pt, Fe
Complete the following reactions
(i) C2H4 + O2 →
(ii) 4Al + 3O2 →
Why does O3 act as a powerful oxidising agent?
How is O3 estimated quantitatively?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area