Surface Chemistry
A colloidal solution of Agl is prepared by two different methods shown below:
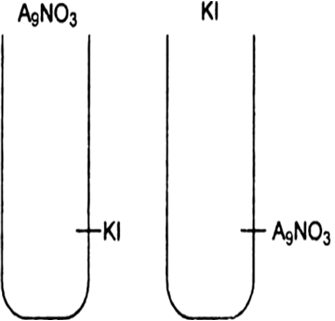
(i) What is the charge of AgI colloidal particles in the two test tube (A) and (B).
(ii) Give reason for the origin of change.
Answer:
When silver nitrate solution is added to potassium iodide solution, negatively charged sol of AgI is formed this due to selective adsorption of I- ion from the dispersion medium.
AgI + I- ---> [AgI]I-
On other hand when KI is added to AgNO3 solution a positively charged sol of AgI is formed this is due to slective adsorption of Ag+ ion present in the dispersion medium.
AgI + Ag+ ---> [AgI]Ag+.
Sponsor Area
Some More Questions From Surface Chemistry Chapter
Why are powdered substances more effective adsorbents than their crystalline forms?
In Haber's process, hydrogen is obtained by reacting methane with steam in presence of NiO as catalyst. The process is known as steam reforming. Why is it necessary to remove CO when ammonia is obtained by Haber’s process?
Why is the ester hydrolysis slow in the beginning and becomes faster after sometime?
What is the role of desorption in the process of catalysis?
Why is it essential to wash the precipitate with water before estimating it quantitatively?
What is adsorption?
What do you mean by adsorbent and adsorbate?
What is desorption?
Define physical and chemical adsorption.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area