Solutions
Question
(a) Draw a labelled diagram to show the change in vapour pressure of a solvent, when a non-volatile solute is added to it.
(b) Show that the change in boiling point of the solvent in this diagram?
Answer
Answer:
(a) It is found that the boiling point of the solution is always higher than that of the pure solvent. The elevation in boiling point is the increase in boiling point when a non-volatile solute is added to a solvent.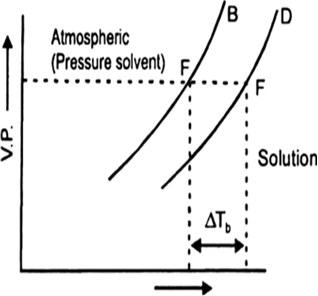
(b) In the above diagram ΔTb indicates the change (increase) in boiling point of solvent.
Sponsor Area
Some More Questions From Solutions Chapter
Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area