Coordination Compounds
Question
Using valence bond theory, predict the shape and magnetic character of [Ni(CO)4] [Ni = 28].
Answer
Ni(28) has outer electronic configuration 4s23d8. The oxidation state of Ni is zero . Thus electronic configuration is 4s°3d10.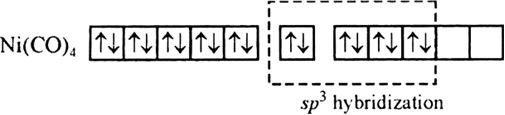
Since there is no any unpaired electron therefore it is diamagnetic in nature and tetrahedral in shape due to sp3 hybridization.
Sponsor Area
Some More Questions From Coordination Compounds Chapter
Write the formula for the following coordination compound:
Tetraaminediaquacobalt(lll)chloride
Write the formula for the following coordination compound:
Potassium tetracyanidonickelate(II)
Write the formula for the following coordination compound:
Tris(ethane -1, 2-diamine) chromium(III) chloride
Write the formula for the following coordination compound:
Amminebromidochloridonitrito-N-platinate (II)
Write the formula for the following coordination compound:
Dichloridobis(ethane-1, 2-diamine)platinum(lV) nitrate.
Write the formula for the following coordination compound:
Iron(III) hexacyanidoferrate(II)
Write the IUPAC names of the following coordination compounds:
(i) [Co(NH3)6]Cl3
(ii) [Co(NH3)5Cl]Cl2
(iii) K3[Fe(CN)6]
(iv) K3[Fe(C2O4)3]
(v) K2[PdCl4]
(vi) [Pt(NH3)2Cl(NH2CH3)]Cl.
Indicate the type of isomerism exhibited by the following complexes and draw structures for these isomers:
(i) K[Cr(H2O)2(C2O4)2], (ii) [Co(en)3Cl3,
(iii) [Co(NH3)5(NO2)]|NO3]2, (iv) [Pt(NH3)(H2O)Cl2]
Explain on the basis of valence bond theory that [Ni(CN)4]2– ion with square planar is diamagnetic and the [NiCl4]2– ion with tetrahedral geometry is paramagnetic.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area