Hydrogen
Compare the structure of H2O and H2O2
(i) In H2O molecule, the central oxygen atom is sp2 hybridised and it is surrounded by two bond pairs and two lone pairs. The presence of two lone pairs brings distortion in the geometry of the molecule. Since the lone pairs repel the bond pairs more effectively resulting in the decrease of H – O - H bond angle from 109.5° to 104.5°, thus water is a bent molecule.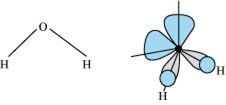
(ii) The structure of H2O2 is like that of an open book. The O – O part of the molecule can be thought of as lying on the spine of a book open at an angle of 90°. The hydrogen atoms are placed one on each cover. The H – O bonds make an angle of about 101.5° with the O – O bond as shown. The O – O linkage present between two oxygen atoms is called peroxide linkage. The structure of hydrogen peroxide in the gas phase and the crystalline state are as shown below.
Sponsor Area
Some More Questions From Hydrogen Chapter
Why does hydrogen occur in a diatomic form rather than in a monoatomic form under normal conditions?
Give one example in which hydrogen acts as an oxidising agent.
Give laboratory Preparation of Dihydrogen ?
Name the substances in which hydrogen exhibits 0 and -1 oxidation states.
Name one isotope of hydrogen which has no neutron.
Which isotope of hydrogen is used as a tracer in organic reactions ?
Hydrogen is the lightest gas. Still its use in filling balloons has been discarded. Why?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area