Redox Reactions
Refer to the periodic table given in your book and now answer the following questions:
(a) Select the possible non-metals that can show disproportionation reaction.
(b) Select three metals that can show disproportionation reaction.
In a disproportionation reaction, one of the reacting substances in a reaction always contains an element that can exist in at least three oxidation states.
(a) The non-metals such as P4,Cl2 and S8 show disproportionation reaction as indicated below:(b) The metals such as Cu+, Ga+, In+ show disproportionation reactions indicated below:
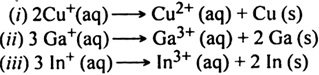
Sponsor Area
Some More Questions From Redox Reactions Chapter
What is reduction (electronic concept)?
What is reducing agent (electronic concept)?
What is an oxidising agent (electronic concept)?
Can oxidation occur without reduction?
What are redox reactions?
What are direct redox reactions? Give one example.
What are indirect redox reactions?
Write formulas for the following compounds:
(a) Mercury (II) chloride
(b) Nickel (II) sulphate
(c) Tin (IV) oxide
(d) Thallium (I) sulphate
(e) Iron (III) sulphate
(f) Chromium (III) oxide
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area