States of Matter
What is hydrogen bond? How is it formed?
A hydrogen bond is defined as the weak electronic force of attraction which exists between the covalently bonded hydrogen of one molecule and highly electronegative atom of another molecule. Hydrogen bond has a strength of the order of 10 – 100 kJ mol–1as compared to another covalent bond which has a strength of the order of 200 – 400 kJ mol–1. A hydrogen bond is denoted by dotted lines (.....). The hydrogen bonding in hydrogen fluoride is represented as:
Cause for the formation of hydrogen bond: Whenever a hydrogen atom is attached to a highly electronegative atom (for example, N, O, F), the shared pair of electrons between the two atoms is attracted towards the more electronegative atom. Consequently, the highly electronegative atom acquires a partial negative charge (δ–) while the hydrogen atom acquires a partial positive charge (δ+). The partial negatively charged atom of one molecule tends to attract partial positive hydrogen atom of the other molecule. This weak electrostatic attraction constitutes hydrogen bond. For example.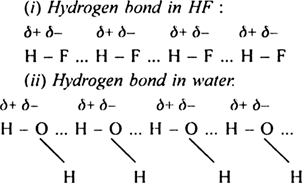
Sponsor Area
Some More Questions From States of Matter Chapter
What is standard (or normal) temperature and pressure (STP)?
What does SATP stand for? Define it.
What is the value of molar volume at STP?
What is standard molar volume?
What is the value of gas constant in SI units?
What is meant by aqueous tension?
What is the nature of gas consant R?
In terms of Charles’s law explain why –273°C is the lowest possible temperature.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area