States of Matter
In terms of Charles’s law explain why –273°C is the lowest possible temperature.
The law states that at constant pressure, the volume of a fixed mass of gas is directly proportional to its absolute temperature. It was found that for all gases (at any given pressure), the plots of volume vs. temperature is a straight line. If this line is extended to zero volume, then it intersects the temperature axis at -2730C.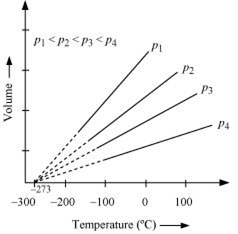
In other words, the volume of any gas at 273 0C is zero. This is because all gases get liquefied before reaching a temperature of 2730C.
Hence, it can be concluded that -273 0C is the lowest possible temperature
Sponsor Area
Some More Questions From States of Matter Chapter
How is the pressure of a gas related to the number of molecules of the gas at constant temperature and volume?
What is standard (or normal) temperature and pressure (STP)?
What does SATP stand for? Define it.
What is the value of molar volume at STP?
What is standard molar volume?
What is the value of gas constant in SI units?
What is meant by aqueous tension?
What is the nature of gas consant R?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area