Chemical Bonding and Molecular Structure
What is hydrogen bond? How is it formed?
A hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom (F, O or N).
Cause of formation of Hydrogen Bond:
When hydrogen is bonded to strongly electronegative element X, the electrons pair shared between the two atoms moves far away from a hydrogen atom. As a result, the hydrogen atoms becomes highly electropositive with respect to the other atom ‘X’. Since there is displacement of electrons towards X, the hydrogen acquires fractional positive charge (δ+) while X attain fractional negative charge (δ–). This result in the formation of a polar molecule having electrostatic force of attraction which can be represented as:
This weak electrostatic attraction constitutes hydrogen bond. For example,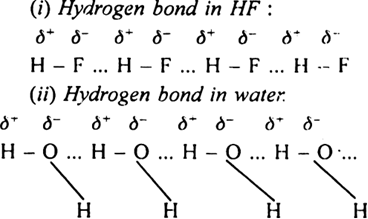
Sponsor Area
Some More Questions From Chemical Bonding and Molecular Structure Chapter
What is crystal lattice?
What is electrovalency?
What bond is present in MgCl2 molecules?
Write Lewis dot symbols for atoms of the elements Mg, Na, B, O, N, Br.
Draw the Lewis structure for the ionic compound by aluminium and fluorine.
Two elements A and B have the electronic configuration as:
A = 1s22s22p63s2 and B = 1s22s22p5
Write the empirical formula of the substance containing A and B.
What type of bonding would you expect between:
(i) a metal and a non-metal
(ii) a non-metal and another non-metal?
Give reasons in one or two sentences for the observation that in their compounds non-metals form anions and not cations.
Out of Na and K, which will form a more stable ionic bond?
Which will have a greater lattice enthalpy: NaCl or MgO?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area