Chemical Bonding and Molecular Structure
Discuss the shape of the following molecules using VSEPR model:
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
BeCl2:
Lewis dot structure Cl: Be : Cl. The central atom (Be) has only two bond pairs and no lone pair. Hence shape is linear.
BCl3:
The central atom (B) has only three bond pairs and no lone pair. Hence shape is triangular planar.
SiCl4:
The central atom (Si) has four bond pairs and no lone pair. Hence the shape is tetrahedral.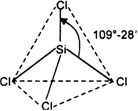
AsF5:
The central atoms (As) has five bond pairs and no lon∈ pair. Hence, the shape is trigonal bipyramidal.
H2S:
The central atom (S) has two bond pairs and two lone pairs. Hence, the shape is Bent or V-shaped.
PH3:
The central atom (P) has three bond pairs and two lone pairs. Hence, the shape is. Bent or V-shaped.
Sponsor Area
Some More Questions From Chemical Bonding and Molecular Structure Chapter
Name the conditions for the formation of an ionic bond between two atoms.
What is crystal lattice?
What is electrovalency?
What bond is present in MgCl2 molecules?
Write Lewis dot symbols for atoms of the elements Mg, Na, B, O, N, Br.
Draw the Lewis structure for the ionic compound by aluminium and fluorine.
Two elements A and B have the electronic configuration as:
A = 1s22s22p63s2 and B = 1s22s22p5
Write the empirical formula of the substance containing A and B.
What type of bonding would you expect between:
(i) a metal and a non-metal
(ii) a non-metal and another non-metal?
Give reasons in one or two sentences for the observation that in their compounds non-metals form anions and not cations.
Out of Na and K, which will form a more stable ionic bond?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area