Classification of Elements and Periodicity in Properties
Nitrogen and phosphorus have high value of ionisation enthalpies. Explain.
The electronic configurations of N (Z = 7) and P(Z = 15) are: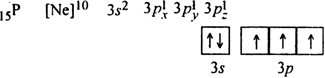
Both these elements have the configurations in which all the orbitals of the same sub-level are exactly half-filled. Such an arrangement gives extra stability to the atom. As a result, the removal of electron becomes difficult and hence they have high value of ionisation enthalpies.
Sponsor Area
Some More Questions From Classification of Elements and Periodicity in Properties Chapter
Give the ‘liquid elements’ in the periodic table.
What is valency of an element?
Gallium was named by Mendeleev as
What are magic numbers?
Which property is the basis of long form of periodic table?
How many elements are placed in each period of the p-block?
What is the number of groups in: (i) p-block (ii) d-block?
How many elements are present in:
(i) second period
(ii) fourth period
(iii) sixth period?
What is the name given to the s-block elements?
To which block the element with outer electronic configuration 4s23d10 belongs?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area