Classification of Elements and Periodicity in Properties
How many blocks are present in the long form of periodic table? How will you classify the elements of the periodic table into s, p, d and f blocks?
The long form of periodic table has been divided into four blocks. These are called:
(i) s-block (ii) p-block
(iii) d-block , (iv) f-block.
Division of elements into s, p, d and f-blocks: This division is based upon the name of the orbital which receives the last electron.
1. s-block elements. The elements in which the last electron enters the s-orbital of the outermost enthalpy level are called .s-block elements. The general electronic configuration of s-block elements is ns1-2 where n stands for the outermost shell. In s-block, the alkali metals of group 1 and alkaline earth metals of group 2 are included.
2. p-block elements: The elements in which the last electron enters the p-orbital of the outermost enthalpy level are called p-block elements. The general electronic configuration of p-block elements in ns2np1-6 where n stands for the outermost shell. The elements of Groups 13, 14, 15, 16, 17, 18 having 3, 4, 5, 6, 7 and 8 electrons respectively in the outermost enthalpy levels constitute p-block elements.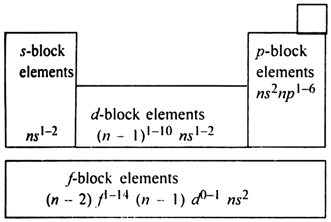
3. d-block elements:The elements in which the last electron enters the d-orbital of the penultimate enthalpy level (last but one shell) are called d-block elements. General electronic configuration of d-block elements is (n - 1) d1-10 ns1-2 where n represents the valence shell, d-block elements have three complete series of ten elements in each whereas the fourth series is incomplete.
Note, Exception is 46Pd whose configuration is 4d10 550:
4. f-block elements:The elements in which the last electron enters the f-orbital of the anti-penultimate (third to the outermost shell) enthalpy level are called f-block elements. The general electronic configuration of f-block elements is (n -2) f1-14 (n-1) d0-1 ns2where n represents the outermost shell. Such type of elements have two series. First is 4f series which is also called lanthanoides series whereas second is 5f series which is also described as actinoide series. These two series are placed separately at the bottom of the periodic table. The elements included in these two series are called inner transition elements.
Sponsor Area
Some More Questions From Classification of Elements and Periodicity in Properties Chapter
What is the number of groups in: (i) p-block (ii) d-block?
How many elements are present in:
(i) second period
(ii) fourth period
(iii) sixth period?
What is the name given to the s-block elements?
To which block the element with outer electronic configuration 4s23d10 belongs?
Why are there 10 elements in each series of d-block?
In terms of electronic configuration,
what is common in a given period and group?
Give the general electronic configuration of
(i) Transition elements (ii) Inner transition elements.
Name the group and period to which an element with Z = 15 belongs ?
What are the advantages of periodic classification of elements?
In the modern periodic table, the period indicates the value of:
(a) atomic number
(b) atomic mass
(c) principal quantum number
(d) azimuthal quantum number.
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area