Structure of Atom
What do you mean by electromagnetic spectrum?
There are many types of electromagnetic radiations such as cosmic rays, gamma rays, X-rays, ultraviolet, visible light, infrared light, microwaves and radio waves. All electromagnetic waves have the same speed (3 X 108 ms-1). However, different types of radiations differ from one another in their wavelengths and therefore in frequency. The arrangement of different types of electromagnetic radiations in the order of increasing wavelengths or decreasing frequencies is known as electromagnetic spectrum. Different regions of the electromagnetic spectrum are identified by different names. Complete electromagnetic spectrum is as shown.
The visible light in the presence of which our eye can see certain radiations is having wavelengths between 380 nm to 760 nm. Different colours in the visible light correspond to radiations of different wavelengths.
Out of the various colours in the visible range. Violet colour corresponds to radiation of minimum wavelength (380 nm) while the red colour corresponds to radiation of maximum wavelength (760 nm)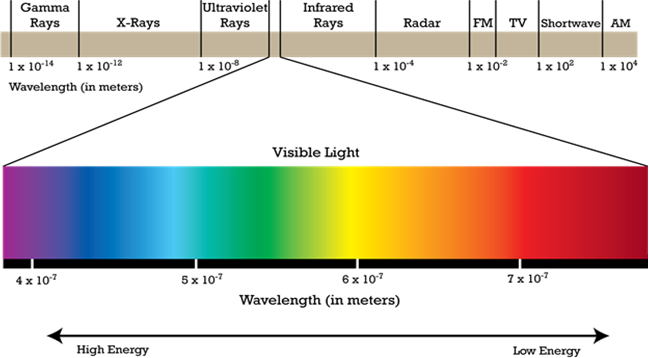
Sponsor Area
Some More Questions From Structure of Atom Chapter
Who discovered neutron?
What is a neutron?
What is the name of the remaining part of atom except outer orbit?
Name the particles which determine the mass of an element.
What are α-particles?
Why were neutrons discovered very late?
What are the fundamental particles present in a neutral atom having atomic number greater than 1?
Do protons and neutrons have identical mass?
When α-particles are sent through a thin metal foil, most of them go straight through the foil. What inference do you draw from it?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area