Organic Chemistry – Some Basic Principles and Techniques
What is sublimation?
Sublimation is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase.
Certain solid substances like naphthalene or camphor when heated pass directly from solid to the vapour state without melting. The vapours, when cooled, give back the solid substance. This process is known as sublimation. The process is very helpful in separating a volatile solid from a non-volatile solid. The powdered substance is taken in a china dish and covered with a perforated filter paper and an inverted funnel. The dish is carefully heated on a sand bath. The vapours passing through the holes in the paper condense on the inner sides of the funnel. The non-volatile impurities remain in the dish.Sublimation is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase.
Sponsor Area
Some More Questions From Organic Chemistry – Some Basic Principles and Techniques Chapter
Which characteristic is common to different isomers of a compound?
Name three alkanes which do not show chain isomerism.
Name the types of structural isomerism shown by alkanes.
Name the four main types of structural isomerism.
Write the three possible open chains of five carbon atoms
What types of structural isomerism is shown by the following pairs of organic compounds ?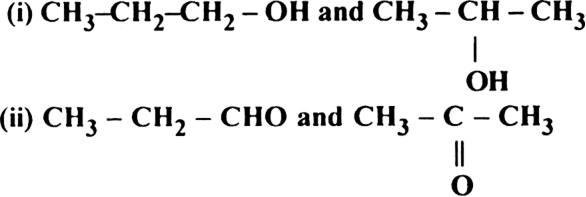
Write the tautomer of acetaldehyde and write its IUPAC name.
Draw the structure of the tautomer of phenol and write its IUPAC name.
Define ring - chain isomerism. Given one example.?
Write the metamer of diethyl ether ?
Mock Test Series
Sponsor Area
NCERT Book Store
NCERT Sample Papers
Sponsor Area